Curanep
Curanep (Nepafenac Mfr. Specs. 0.1%) by Schazoo are eye drops. It’s a sterile ophthalmic suspension, ocular non-steroidal & anti-inflammatory/analgesic (NSAIDs) 5 ml liquid FOR OPHTHALMIC USE ONLY. Nepafenac is designated chemically as 2-amino-3-benzoylbenzeneacetamide.
After the drops go into the eye, nepafenac is changed into amfenac, which works by blocking the chemicals that cause the eye’s pain and inflammation.
Package Contains: Each Curanep package contains eye drops in a 5ml plastic dropper bottle & a leaflet.
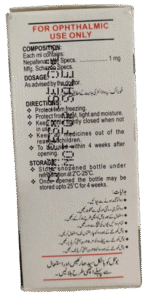
Composition: Each ml contains: 
Nepafenac: Specs. 1 mg
Mfg. Schazoo Specs.
Dosage: As directed by the doctor.
Indication & Usage: Curanep sterile ophthalmic suspension is indicated for the treatment of pain and inflammation associated with cataract surgery.
Dosage & Administration: One drop of Curanep sterile ophthalmic suspension should be applied to the affected eye(s) three times daily beginning 1 day prior to cataract surgery, continued on the day of surgery, and through the first 2 weeks of the postoperative period.
Curanep sterile ophthalmic suspension may be administered in conjunction with other topical ophthalmic medications such as beta-blockers, carbonic anhydrase inhibitors, alpha-agonists, cycloplegics, and mydriatics.
Contraindications: Curanep sterile ophthalmic suspension is contraindicated in patients with previously demonstrated hypersensitivity to any of the ingredients in the formulation or to other NSAIDs.
Precautions & Warnings:
Warnings: There is the potential for cross sensitivity to acetylsalisylic acid, phenylacetic acid derivatives, and other nonsteroidal anti-inflammatory agents. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
With some nonsteroidal anti-inflammatory drugs, including Curanep, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied nonsteroidal anti-inflammatory drugs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
Precautions: General: Topical nonsteroidal anti-inflammatory drugs (NSAIDs), including Curanep, may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration or corneal perforation. These events may be sight-threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs, including Curanep, and should be closely monitored for corneal health.
Post-marketing experience with topical NSAIDs suggests that patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events, which may become sight-threatening. Topical NSAIDs should be used with caution in these patients.
Post marketing experience with topical NSAIDs also suggests that use for more than 1 day prior to surgery or use beyond 14 days post-surgery may increase patient risk and severity of corneal adverse events.
It is recommended that Curanep sterile ophthalmic suspension be used with caution in patients with known bleeding tendencies or who are receiving other medications that may prolong bleeding time.
Information for Patients: Curanep sterile ophthalmic suspension should not be administered while using contact lenses.
Use in Pregnancy: Curanep sterile ophthalmic suspension should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Non-teratogenic Effects: Because of the known effects of prostaglandin biosynthesis-inhibiting drugs on the fatal cardiovascular system (closure of the ductus arteriosus), the use of Curanep during late pregnancy should be avoided.
Nursing mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Curanep sterile ophthalmic suspension is administered to a nursing woman.
Pediatric use: The safety and effectiveness of Curanep sterile ophthalmic suspension in pediatric patients below the age of 10 years have not been established.
Adverse Reactions: The most frequently reported ocular adverse events following cataract surgery are capsular opacity, decreased visual acuity, foreign body sensation, increased intraocular pressure, and sticky sensation. These events occur in approximately 5 to 10% of patients.
Other ocular adverse events occurring at an incidence of approximately 1 to 5% including conjunctival edema, corneal edema, dry eye, lid margin crusting, ocular discomfort, ocular hyperemia, ocular pain, ocular pruritus, photophobia, tearing and vitreous detachment.
Some of these events may be the consequence of the cataract surgical procedure. Non-ocular adverse events reported at an incidence of 1 to 4% include headache, hypertension, nausea/vomiting, and sinusitis.
Directions: Protect from freezing. Protect from heat, light, and moisture. Keep the bottle tightly closed when not in use. Keep all medicines out of the reach of children. To be used within 4 weeks after opening. To be sold on prescription of a registered medical practitioner only.
STORAGE: Store the unopened bottle under refrigeration at 2°C-25°C. Once opened, the bottle may be stored up to 25°C for 4 weeks.
Manufacturer & Buy Online:
Curanep eye drops are manufactured by: The Schazoo Pharmaceutical Laboratories (Pvt) Ltd.
This is a link to the manufacturer at: https://schazoo
You can buy this medicine online at: https://healthwire.pk